Fact checkers
Fact checkers
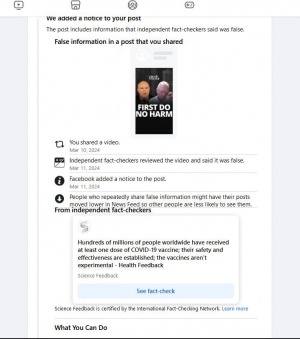
Fact checkers are often misleading and deceptive in their presentations and there are often not independent but are paid by those spreading false narratives.
Here is one that is shameless in their efforts to press forward with their propaganda.
The factcheck below was Published on: 22 Jan 2022 | Editor: Flora Teoh
You be the judge:
Hundreds of millions of people worldwide have received at least one dose of COVID-19 vaccine; their safety and effectiveness are established; the vaccines aren’t experimental
"Claims that the COVID-19 vaccines are experimental and/or that vaccinated people are guinea pigs aren’t new. They circulated early in 2020, as this previous Health Feedback review reported. At that time, three COVID-19 vaccines (Pfizer-BioNTech, Moderna, Johnson & Johnson) were authorized for use under the U.S. Food and Drug Administration’s Emergency Use Authorization program. Since then, the Pfizer-BioNTech COVID-19 vaccine received FDA approval. Nevertheless, the claim that the vaccines are experimental proves to be popular, as multiple Health Feedback reviews about the same claim in viral social media posts by personalities such as another political commentator Candace Owens and chiropractor Benjamin Benulis, show.
The safety and effectiveness of COVID-19 vaccines were established in clinical trials before receiving EUA
The FDA describes the concept of an EUA as follows:
- “Under an EUA, FDA may allow the use of unapproved medical products, or unapproved uses of approved medical products in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain statutory criteria have been met, including that there are no adequate, approved, and available alternatives.”
Wikipedia
"An Emergency Use Authorization (EUA) in the United States is an authorization granted to the Food and Drug Administration (FDA) under sections of the Federal Food, Drug, and Cosmetic Act as added to and amended by various Acts of Congress, including by the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 (PAHPRA), as codified by 21 U.S.C. § 360bbb-3, to allow the use of a drug prior to approval.[1] It does not constitute approval of the drug in the full statutory meaning of the term, but instead authorizes the FDA to facilitate availability of an unapproved product, or an unapproved use of an approved product, during a declared state of emergency from one of several agencies or of a "material threat" by the Secretary of Homeland Security."